AZD-9291: Advanced Inhibitor for EGFR T790M Positive Non-Small Cell Lung Cancer
A targeted therapy breakthrough for patients with EGFR T790M mutation-positive metastatic NSCLC.
Get a Quote & SampleProduct Core Value
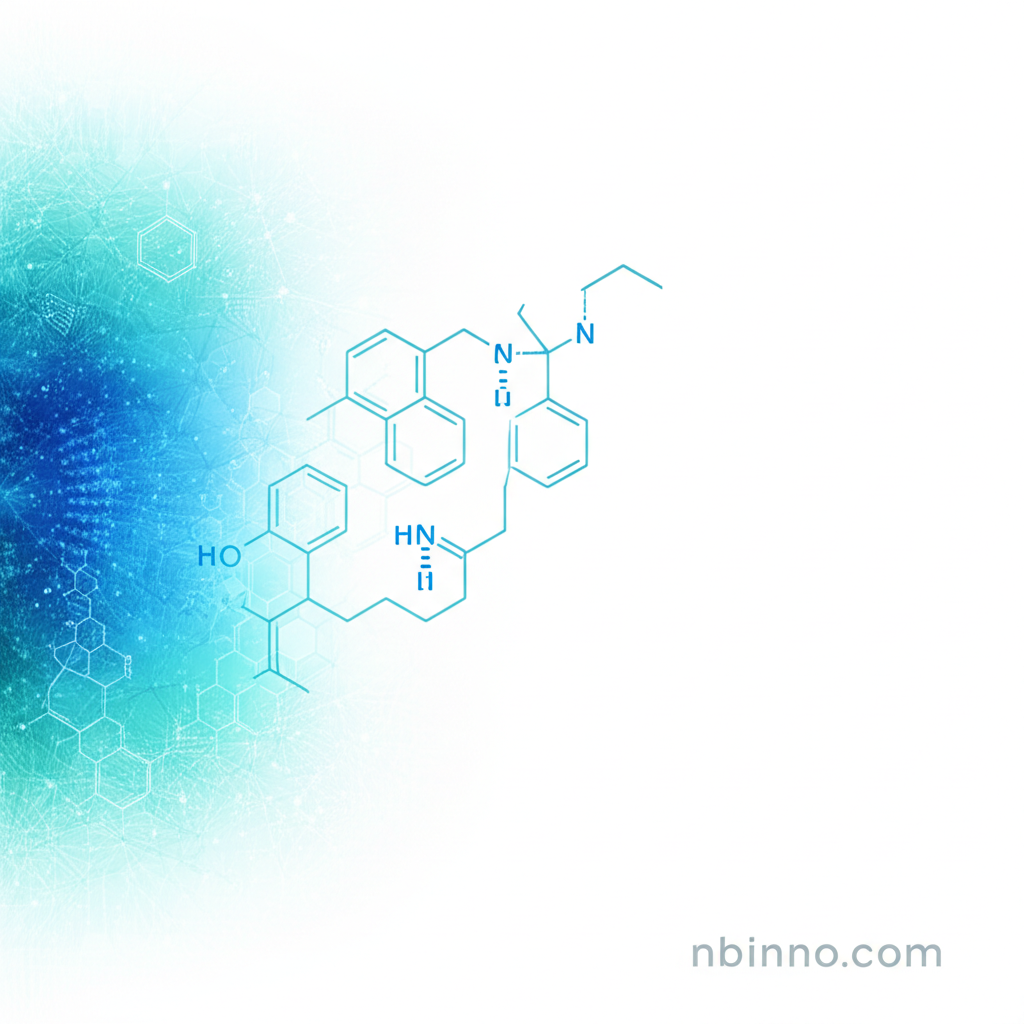
AZD-9291
AZD-9291, also known as Osimertinib, is a crucial targeted therapy designed for patients diagnosed with metastatic non-small cell lung cancer (NSCLC) who have the specific EGFR T790M mutation. This medication plays a vital role in overcoming resistance that often develops after patients have undergone treatment with prior Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors (EGFR-TKIs).
- Discover targeted therapy for EGFR T790M positive NSCLC, offering a new lifeline to patients who have developed resistance to previous treatments.
- Osimertinib for metastatic NSCLC provides a sophisticated approach to managing advanced stages of the disease.
- Understand how AZD-9291 addresses EGFR TKI resistance treatment, a common challenge in lung cancer therapy.
- Learn about the clinical advancements in lung cancer treatment with AZD9291, supported by robust trial data like the AURA3 study.
Advantages of AZD-9291
Targeted Mechanism
AZD-9291 is specifically designed to inhibit both the activating EGFR mutations and the T790M resistance mutation, providing a precise treatment for identified patient profiles.
Overcoming Resistance
It offers a vital solution for patients whose tumors have developed resistance to earlier EGFR-TKIs, a common issue in the T790M mutation positive lung cancer therapy.
Clinical Efficacy
Supported by significant clinical trial data, AZD-9291 demonstrates compelling efficacy, including high objective response rates, in the intended patient population.
Key Applications
Oncology Treatment
Primarily used in the treatment of metastatic non-small cell lung cancer (NSCLC) characterized by the presence of the T790M mutation, as part of precision medicine in oncology.
Overcoming TKI Resistance
A critical application is to provide an effective treatment option for patients who have become resistant to first-generation EGFR-TKIs, a key aspect of advances in targeted lung cancer therapies.
Clinical Research
AZD-9291 is extensively studied in clinical trials, such as AURA3, to further understand its benefits and potential in various NSCLC scenarios, contributing to Osimertinib patient education.
Targeted Drug Development
Represents a significant advancement in the field of targeted drug development for specific genetic mutations driving cancer progression, crucial for pharmaceuticals for respiratory cancers.
