High-Purity Sacubitril Impurity C (CAS 149709-63-7) Manufacturer & Supplier in China
Discover the leading manufacturer and supplier of Sacubitril Impurity C (CAS 149709-63-7) in China. We offer high-quality pharmaceutical intermediates for API development, analytical method validation, and quality control. Partner with us for reliable supply and competitive pricing.
Get a Quote & SampleYour Trusted Source for Sacubitril Impurity C
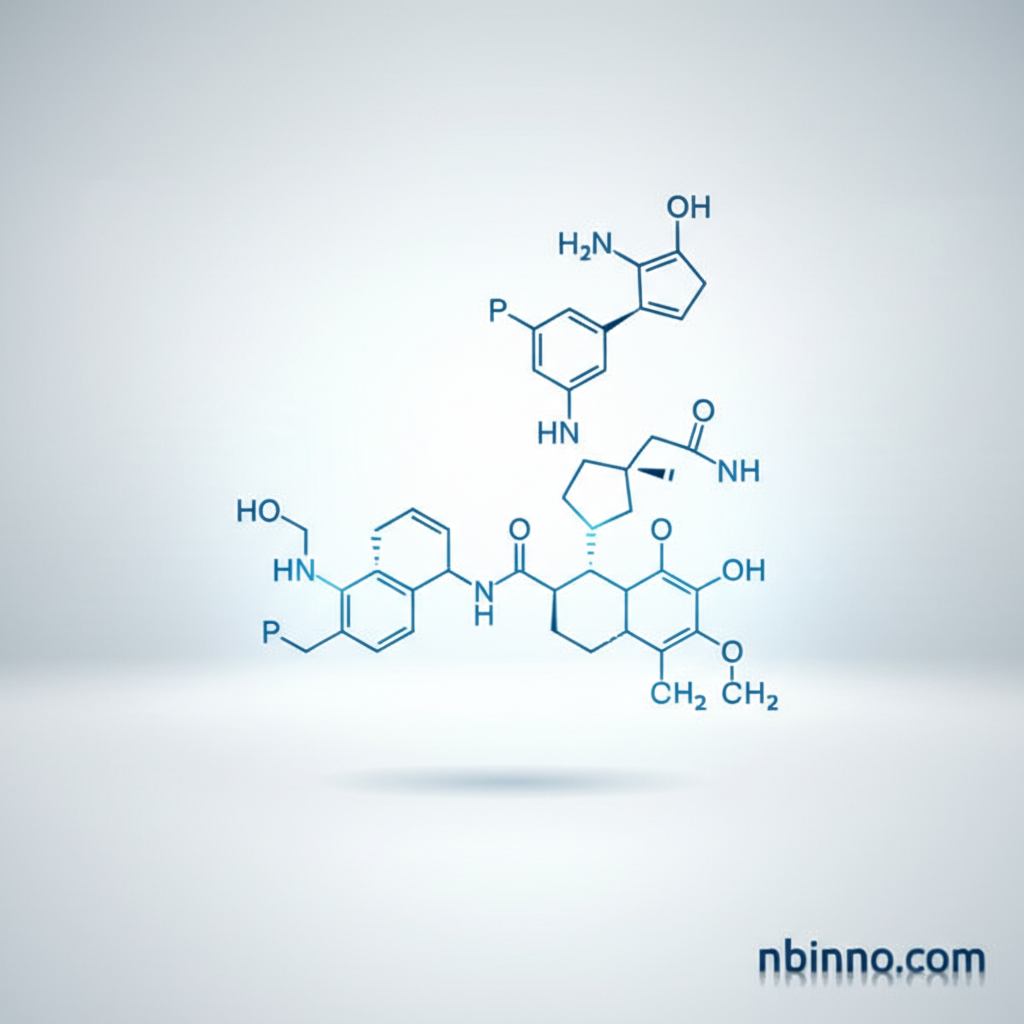
Sacubitril Impurity C (CAS 149709-63-7)
As a premier manufacturer and supplier of pharmaceutical intermediates, we specialize in providing high-purity Sacubitril Impurity C (CAS 149709-63-7). This critical compound is essential for API manufacturers and researchers involved in drug discovery and quality assurance. Our commitment to excellence ensures that you receive a product that meets stringent quality standards, making us a reliable partner for your pharmaceutical development needs. Buy with confidence from our China-based facility.
- High-Purity Sacubitril Impurity C for API Manufacturers
- CAS 149709-63-7: Essential for Analytical Method Development and Validation
- Reliable Supplier in China with ISO Certification for Consistent Quality
- Competitive Pricing and Bulk Order Availability for Purchase Inquiries
Key Advantages of Our Sacubitril Impurity C
Uncompromising Purity and Quality
Our Sacubitril Impurity C (CAS 149709-63-7) is produced under strict quality control measures, ensuring high purity crucial for pharmaceutical applications and reliable results in your research and development projects.
Critical for Pharmaceutical Development
This impurity standard is vital for API manufacturers for method development, validation, and quality control processes, ensuring the safety and efficacy of final drug products. Inquire about purchasing this key intermediate.
Global Supply Chain Expertise
Leveraging our extensive experience as a manufacturer and supplier, we offer a robust supply chain to meet your demands for Sacubitril Impurity C, providing efficient delivery worldwide. Contact us for a quote.
Applications of Sacubitril Impurity C
Analytical Method Development
Utilize Sacubitril Impurity C (CAS 149709-63-7) to develop and validate sensitive analytical methods, such as HPLC, for detecting and quantifying impurities in Sacubitril formulations.
Quality Control
Essential for quality control processes in pharmaceutical manufacturing, ensuring that the finished drug products meet regulatory specifications and purity requirements.
API Research and Production
As a key intermediate, it plays a role in the synthesis and characterization of Active Pharmaceutical Ingredients (APIs), supporting R&D and commercial production.
Reference Standard
Serve as a reliable reference standard for researchers and manufacturers, providing traceability and accuracy in laboratory testing and quality assessments.
